- Home
- About ACA
- Publications & Resources
- Programs
- Annual Meeting
- Membership
- ACA History Center
- Media Archive
ACA Early Career Scientist Spotlight-23Personal StatementI became interested in structural biology as an undergraduate researcher working on essential proteins of Mycobacterium tuberculosis. One of the graduate students in the lab pulled me over to his computer to show me what looked like a video game but what I would later come to understand was a protein structure and electron density being manipulated in Coot. I was instantly intrigued, and eager to solve my own structure, setting me on the course to pursue a career as a structural biologist. In graduate school at the University of Toledo under the mentorship of Prof. Timothy Mueser I studied pyridoxal 5’-phosphate (PLP)-dependent enzymes using neutron diffraction. PLP-dependent enzymes utilize the phosphorylated vitamin B6 derivative to carry out a variety of different chemistries including transamination, racemization, phosphorylation, α-decarboxylation, aldol cleavage, β- and γ-elimination and replacement reactions, and glycogen phosphorylation. This family of enzymes is present in all forms of life and predominantly located in amino acid metabolic pathways. To understand how PLP-dependent enzymes achieve their reaction specificity and versatility requires knowledge of the location of all atoms in the structure, including hydrogen. Neutron diffraction allows for direct visualization of hydrogen (or deuterium) atoms at moderate resolutions. The bottleneck of this technique is the need for large (>0.5 mm3) crystals to overcome the low flux of neutron beamlines.
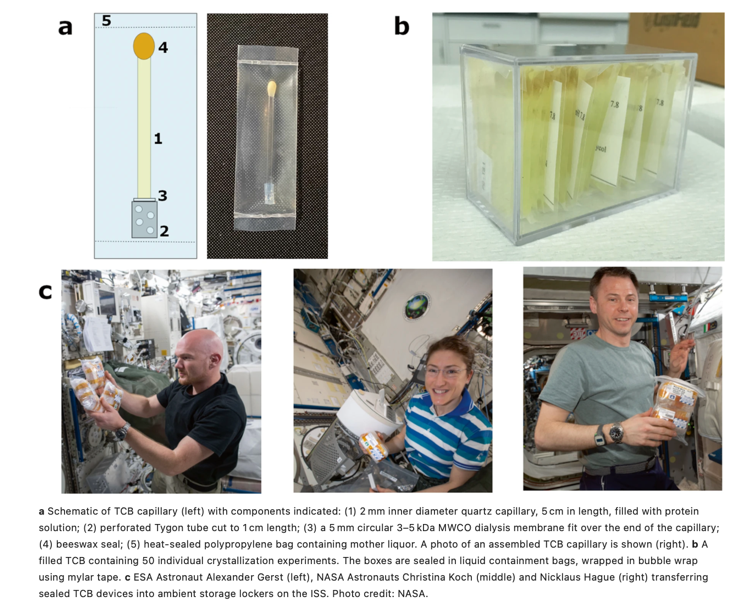
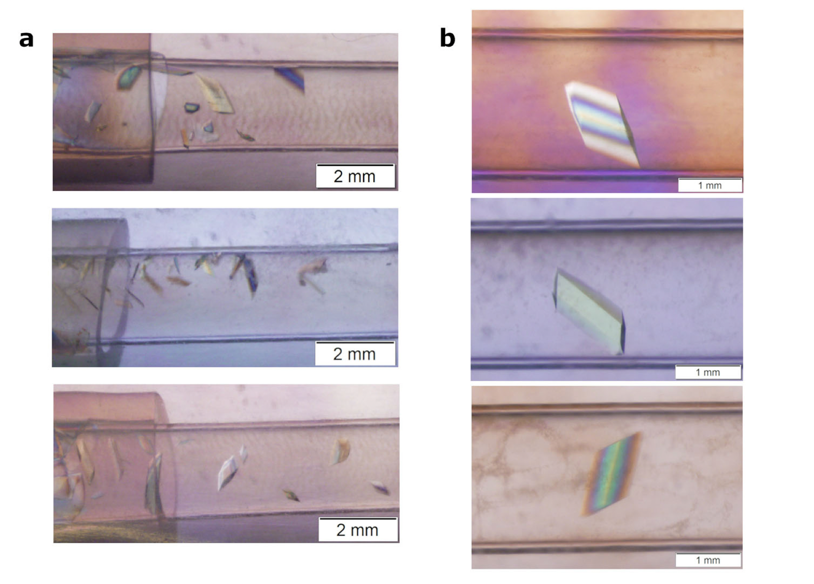
After a multitude of failed attempts of acquiring large, single crystals of a PLP-dependent enzyme, tryptophan synthase (TS), we turned to the sky (literally) for a solution. We developed the Toledo Crystallization Box (TCB) for microgravity crystallization for neutron diffraction. The lack of gravity-driven convection promotes uniform crystal feeding and packing, which can improve the quality and size of the crystal. The TCB, which utilizes slow-equilibration capillary dialysis crystallization, was implemented on two Protein Crystal Growth (PCG) initiatives aboard the International Space Station (ISS) sponsored by the Center for Advancement of Science in Space (CASIS). In June 2018, the TCB apparatus was tested aboard ISS carrying 150 protein crystallization experiments for PCG-8. The one-month duration of this mission was not long enough to allow the capillaries to fully equilibrate. In July 2019, we sent the TCBs to the ISS for a second time with the PCG-15 initiative with a longer, 6-month duration in microgravity. We observed a 5-fold increase in size in the crystals grown in microgravity compared to ground control. The neutron diffraction of the crystals was compared using beamline IMAGINE at Oak Ridge National Laboratory. Microgravity-grown and ground control of 0.2 mm3 size diffracted neutron to 3.2 and 4.0 Å, respectively. While 0.2 mm3 is smaller than what would normally be used in a neutron diffraction experiment, the resolution is correlated with the size of the crystal and therefore could be biased by using different sized crystals. Crystals grown in microgravity (>1 mm3) were sent to Institut Laue-Langevin in Grenoble, France where a 2.1 Å neutron dataset was collected on LADI-III. Development and implementation of the TCB was recently published.
Since graduating, I have accepted the role of a Postdoctoral Research Associate at ORNL in the Neutron Scattering Division. Under the mentorship of Andrey Kovalevsky, I continue to use neutron diffraction to study PLP-dependent enzymes, primarily serine hydroxymethyltransferase (SHMT). SHMT catalyzes the cleavage of the C-C bond in serine to produce glycine and formaldehyde, which subsequently undergoes condensation with tetrahydrofolate (THF) to produce 5,10-methyleneTHF. This reaction provides single carbon units for one-carbon metabolism, necessary for the synthesis of methionine and DNA nucleobases. As a result, SHMT is essential for life and has been identified as an important pharmaceutical target for antibiotics and antiprotozoals. In humans, both a cytosolic (SHMT1) and mitochondrial (SHMT2) isoforms of the enzyme exist. SHMT2 is overexpressed in cancer cells and has become a significant target for the development of cancer therapeutics. We are studying SHMT from two bacterial organisms, Thermus thermophilus and Mycobacterium tuberculosis, as well as the human mitochondrial isoform. To date, we have collected two neutron diffraction datasets on T.th. SHMT exhibiting two different states in the reaction mechanism. Because the active site of SHMT is highly conserved between organisms, knowledge of the structure and mechanism of bacterial SHMT can be applied to the human enzyme. This research furthers the understanding of PLP modulation by PLP-dependent enzymes and advance the development of SHMT-targeted pharmaceuticals. |